Solubility Tempterature Lab Gizmo : Mr Evangelos' Regent's Chemistry: Lab 10.2 - Solubility vs ... - Increasing pressure increases the solubility of a.
Solubility Tempterature Lab Gizmo : Mr Evangelos' Regent's Chemistry: Lab 10.2 - Solubility vs ... - Increasing pressure increases the solubility of a.. When a solid dissolves in a liquid, a change in the physical state of the solid analogous to melting case i: Temperature and solubility worksh… student exploration solubility and te… solubility and temperature lab. · study the effect of changing temperature on the amount of solute that will dissolve in a given amount of water. Increasing pressure increases the solubility of a. Decrease in solubility with temperature:
Solubility and temperature gizmo | explorelearning. Comparing gas solubility in liquids with the concept of vapor pressure highlights another important pattern: The specific heat capacity of a substance is the amount of energy needed to change the temperature of that substance by 1 c. If the heat given off in the dissolving process is greater than the heat required to break apart the solid. You will use the calorimetry lab gizmo to determine the specific heat capacities of various substances.

This is why sugar dissolves better in hot water than in cold water.
In the solubility and temperature gizmo™, you will study how temperature affects how much solute will dissolve in a solution. If the heat given off in the dissolving process is greater than the heat required to break apart the solid. Increasing the temperature by adding heat drives the reaction to the reactant side and decreases solubility. Usually, increasing the temperature increases the solubility of solids and liquids. Then test your prediction by creating the solution in the virtual lab. Concentration, dissolve, homogeneous mixture, solubility, solubility curve, solute, solution, solvent prior knowledge questions (do these before using the gizmo.) You will use the calorimetry lab gizmo™ to determine the specific heat capacities of various 1. When you add a solute to a solvent, the kinetic energy of the solvent molecules overcomes the attractive forces among solute particles. On the simulation pane, select copper. Specific heat capacity can be described as a substance's resistance to temperature changes. So by increasing temperature, you supply a needed reactant in the dissolution reaction. The created in this lab graph was very similar to the one on table g in the reference tables. The solubility of solutes is dependent on temperature.
If the heat given off in the dissolving process is greater than the heat required to break apart the solid. Temperature (see notice how the temperature dependence of nacl is fairly flat. Add varying amounts of a chemical to a beaker of water to create a solution, observe that the chemical dissolves in the water at first, and then measure the concentration of the solution at the saturation point. Comparing gas solubility in liquids with the concept of vapor pressure highlights another important pattern: In general, solids become more soluble as the temperature increases.

Gizmo answers solubility and temperature the temperature dependence of solubility can be visualized with the help of a solubility curve , a graph of the solubility vs.
Increasing the temperature always decreases the solubility of gases. This pdf book contain chemfax lab answers solubility and temperature conduct. Www.explorelearning.com › gizmos chapter 1. · study the effect of changing temperature on the amount of solute that will dissolve in a given amount of water. If the heat given off in the dissolving process is greater than the heat required to break apart the solid. On the simulation pane, select copper. However because we only had four points on our graph it created some room for error. In the solubility and temperature gizmo™, you will study how temperature affects how much solute will dissolve in a solution. When a solid dissolves in a liquid, a change in the physical state of the solid analogous to melting takes place. Solubility and temperature get the gizmo ready: Add varying amounts of a chemical to a beaker of water to create a solution, observe that the chemical dissolves in the water at first, and then measure the concentration of the solution at the saturation point. So by increasing temperature, you supply a needed reactant in the dissolution reaction. The world of physical science section.
Specific heat capacity can be described as a substance's resistance to temperature changes. So by increasing temperature, you supply a needed reactant in the dissolution reaction. In the solubility and temperature gizmo™, you will study how temperature affects how much solute will dissolve in a solution. This property is taken advantage of in its purification. 3 computer lab assignments | tonya harvey.
Concentration, dissolve, homogeneous mixture, solubility, solubility curve, solute, solution, solvent prior knowledge questions (do these before using the gizmo.)
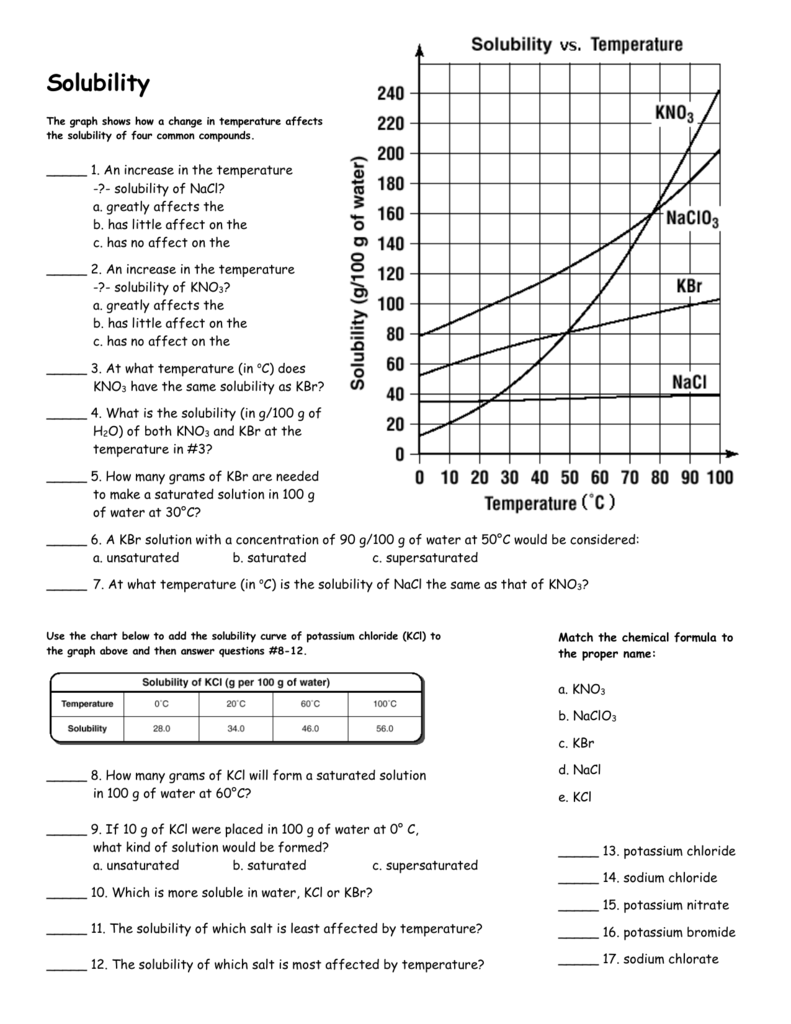
Solubility versus temperature this chart shows the solubility of various substances in water at a variety of temperatures (in degrees celsius). Heat is required to break the bonds holding the molecules in the solid together. You will use the calorimetry lab gizmo™ to determine the specific heat capacities of various 1. This property is taken advantage of in its purification. Is 20 °c and that potassium nitrate is selected. Of the water is 20 °c. Concentration, dissolve, homogeneous mixture, solubility, solubility curve, solute, solution, solvent prior knowledge questions (do these before using the gizmo.) Comparing gas solubility in liquids with the concept of vapor pressure highlights another important pattern: Solubility and temperature gizmo | explorelearning. The created in this lab graph was very similar to the one on table g in the reference tables. When you add a solute to a solvent, the kinetic energy of the solvent molecules overcomes the attractive forces among solute particles. Given the solubility of cucl at 2 different temperatures, predict its solubility at a third temperature. Temperature and solubility worksh… student exploration solubility and te… solubility and temperature lab.
Komentar
Posting Komentar